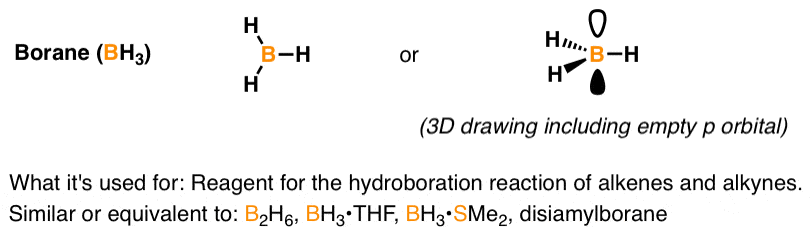
Pka Vinyl Hydrogen

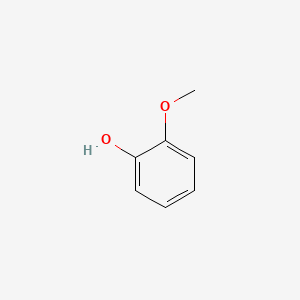
Alcohol pka 16 18 8.
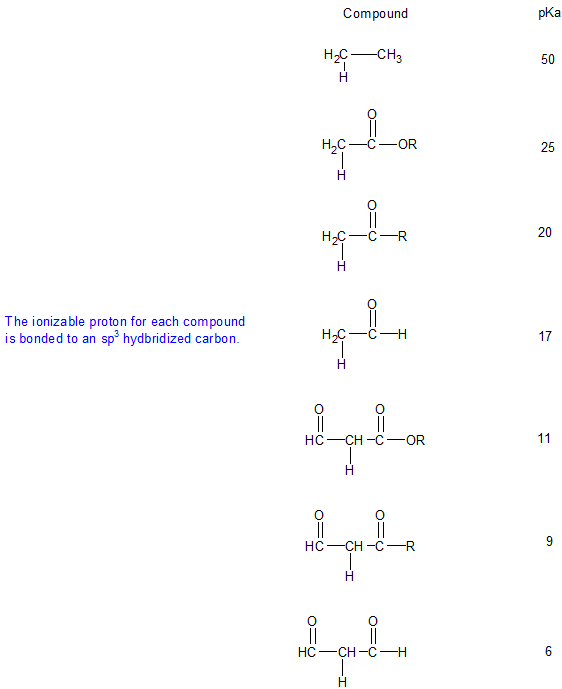
Pka vinyl hydrogen. Electrons in unconjugated sp2 orbital phenyl hydrogens. Electrons in unconjugated sp2 orbital not part of aromatic π sextet hydrogen gas is a weak acid allyl. Aldehyde pk a 17 ketone pk a 19 and an ester pk a 25 and try to justify the trend. Aryl 43 benzylic 41 15.
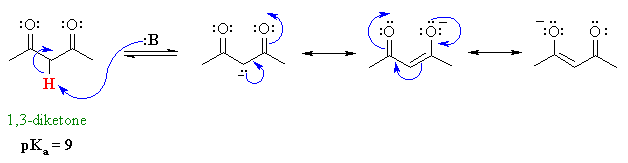
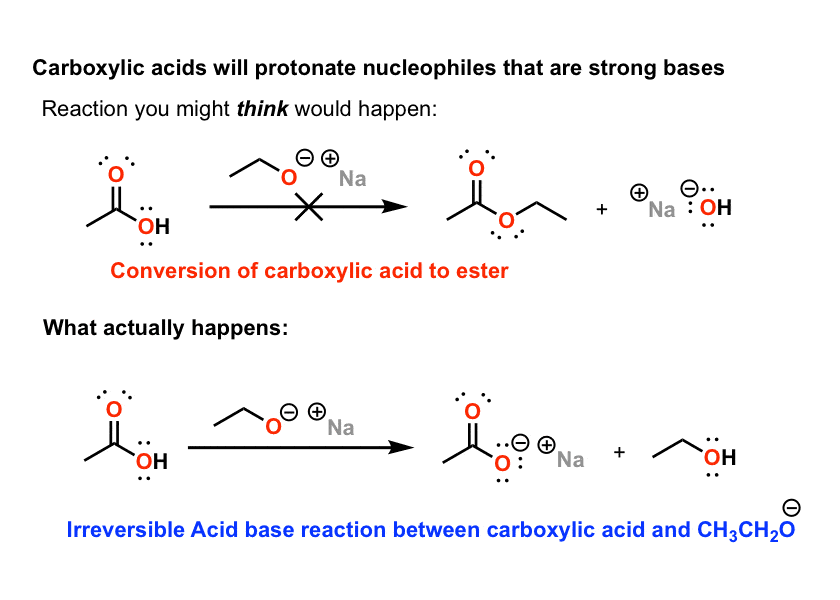
In this reaction accn is a radical initiator and an aliphatic thiol transfers radical character to the silylhydride. Weakest acid on this table. Base methane and hydrogens on sp3carbons cyclopropane h. The difference between the 3 systems is in the nature of the group attached to the common carbonyl.
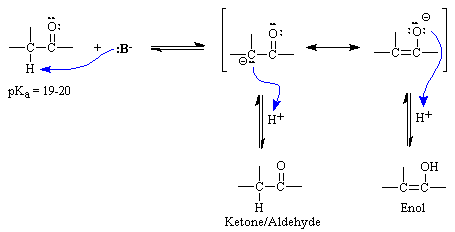
Williams page 1 pka values index inorganic 2 phenazine 24 phosphates 3 pyridine 25 carboxylic acids 4 8 pyrazine 26 aliphatic 4 8 aromatic 7 8 quinoline 27 phenols 9 quinazoline 27 alcohols and oxygen acids 10 11 quinoxaline 27 amino acids 12 special nitrogen compounds 28 peptides 13 hydroxylamines 28. The triethylsilyl free radical then reacts with the azide with expulsion of nitrogen to a. Pka data compiled by r. Alpha proton of ketone aldehyde pka 20 11.
Water pka 15 7 9. Terminal alkyne pka 25 13. More vinyl than sp3 hybrid see below vinyl hydrogens. Let s compare pk a of the common systems.
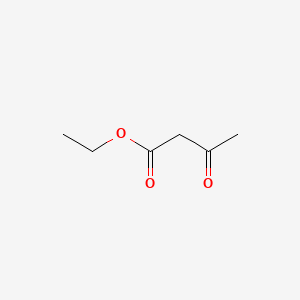
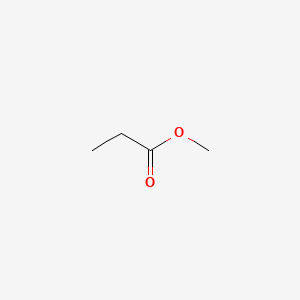
Hydrogen peroxide is a chemical compound with the formula h 2 o 2 in its pure form it is a very pale blue liquid slightly more viscous than water hydrogen peroxide is the simplest peroxide a compound with an oxygen oxygen single bond it is used as an oxidizer bleaching agent and antiseptic concentrated hydrogen peroxide or high test peroxide is a reactive oxygen species and has. In the laboratory silyl hydrides are used as reducing agent for example pmhs in one study triethylsilane is used in the conversion of a phenyl azide to an aniline. Alpha proton of ester pka 25 12.