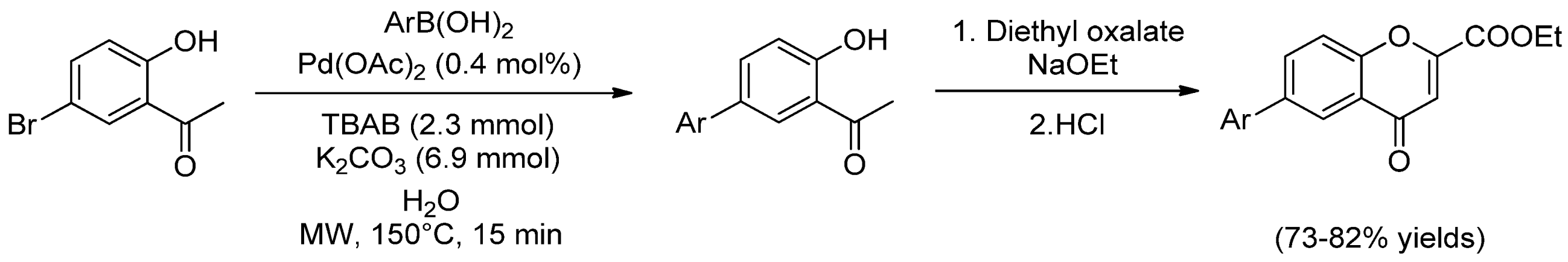
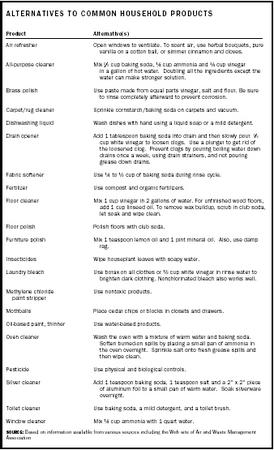
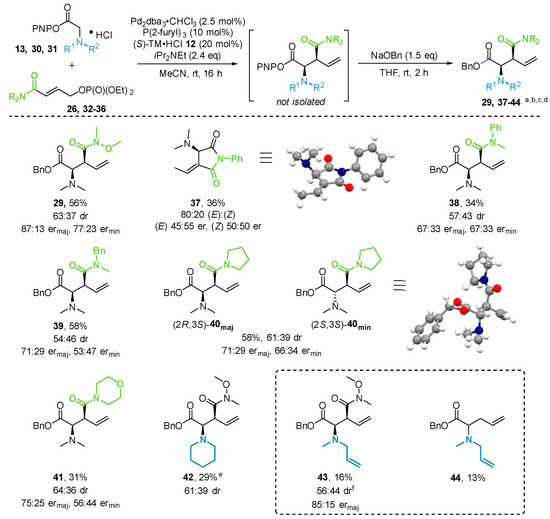
Phenyl Oxalate Ester Floor Cleaner

Phenyl oxalate ester is responsible for the luminescence in a glow stick.
Phenyl oxalate ester floor cleaner. Ethanedioic acid diphenyl ester. When you bend the glow stick and break the inner container the two solutions mix and a chemical reaction initiates between the hydrogen peroxide and diphenyl oxalate ester producing two. Oxalic acid diphenyl ester. Gently mop the floor.
Most glow sticks contain hydrogen peroxide phenyl oxalate ester and a fluorescent dye. Before you attempt to treat the fabric at home with a home dry cleaning kit read the home kit instructions thoroughly prior to use. Phthalates are being phased out in many products due to health concerns. Phthalic ester is a substance that is added to plastics to increase flexibility durability and transparency.
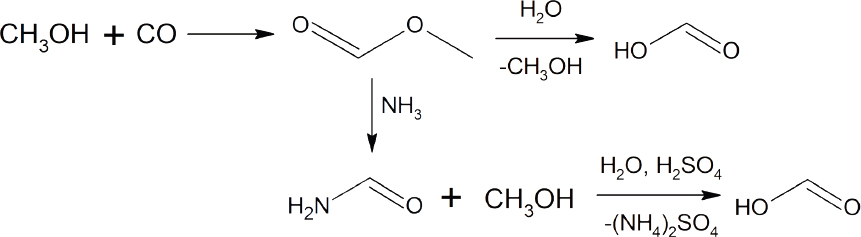
If the stained fabric is dry clean only be sure to point out the glow stick stain to a professional dry cleaner. The present invention discloses a molybdenum oxide catalyst for synthesizing phenyloxalate by an ester exchange process and a preparation process thereof which belongs to a technology for preparing methyl phenyl oxalate and a catalyst of the phenyloxalate wherein the methyl phenyl oxalate is the raw material for synthesizing diphenyl carbonate dpc. It is non toxic and harmless to human beings and hence very safe to use. Oxalic acid diphenyl ester.
Phenyl oxalate ester and fluorescent dyes that produce the stains. By mixing the peroxide with the phenyl oxalate ester a chemical reaction takes place yielding two moles of phenol and one mole of peroxyacid ester 1 2 dioxetanedione. Articles of diphenyl oxalate are included as well. The damage begins with the hydrogen peroxide which is a mild form of bleach that can remove the original color from fabrics and the fluorescent dyes usually green red or blue will stain.
The dioxetanedione then reacts with a dye molecule decomposing to form carbon dioxide and. The chemicals inside the plastic tube are a mixture of the dye the base catalyst and diphenyl oxalate. The reaction with hydrogen peroxide causes the liquid inside a glow stick to glow. The chemical in the glass vial is hydrogen peroxide.
Pour a cap full 9 12 ml of fly go in a half bucket of water 5 6 ltr. Diphenyl oxalate trademark name cyalume is a solid whose oxidation products are responsible for the chemiluminescence in a glowstick this chemical is the double ester of phenol with oxalic acid upon reaction with hydrogen peroxide 1 2 dioxetanedione is formed along with release of the two phenols. No need to rinse.