Pharmaceutical Clean Room Qualification

This article is featured in the may 2019 issue of cleanroom technology.
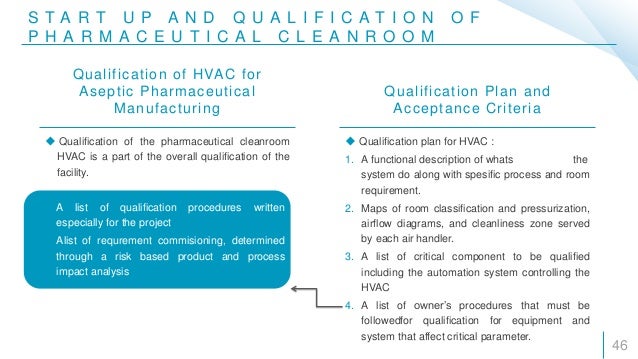
Pharmaceutical clean room qualification. Certification procedures iq and oq. To confirm the purpose of the pharmaceutical cleanroom to discuss the relevant guidelines and regulations for pharmaceutical cleanroom commissioning certification and validation to discuss the cleanroom validation procedures 1. Commissioning and qualification are critical steps in the pharmaceutical drug and biologic supply chain process. A strong and comprehensive qualification increases the company s regulatory compliance position enhances product quality and ensures patient safety.
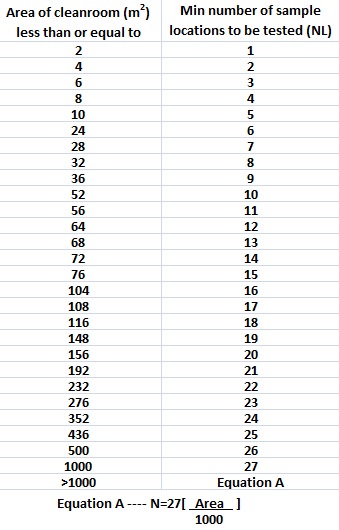
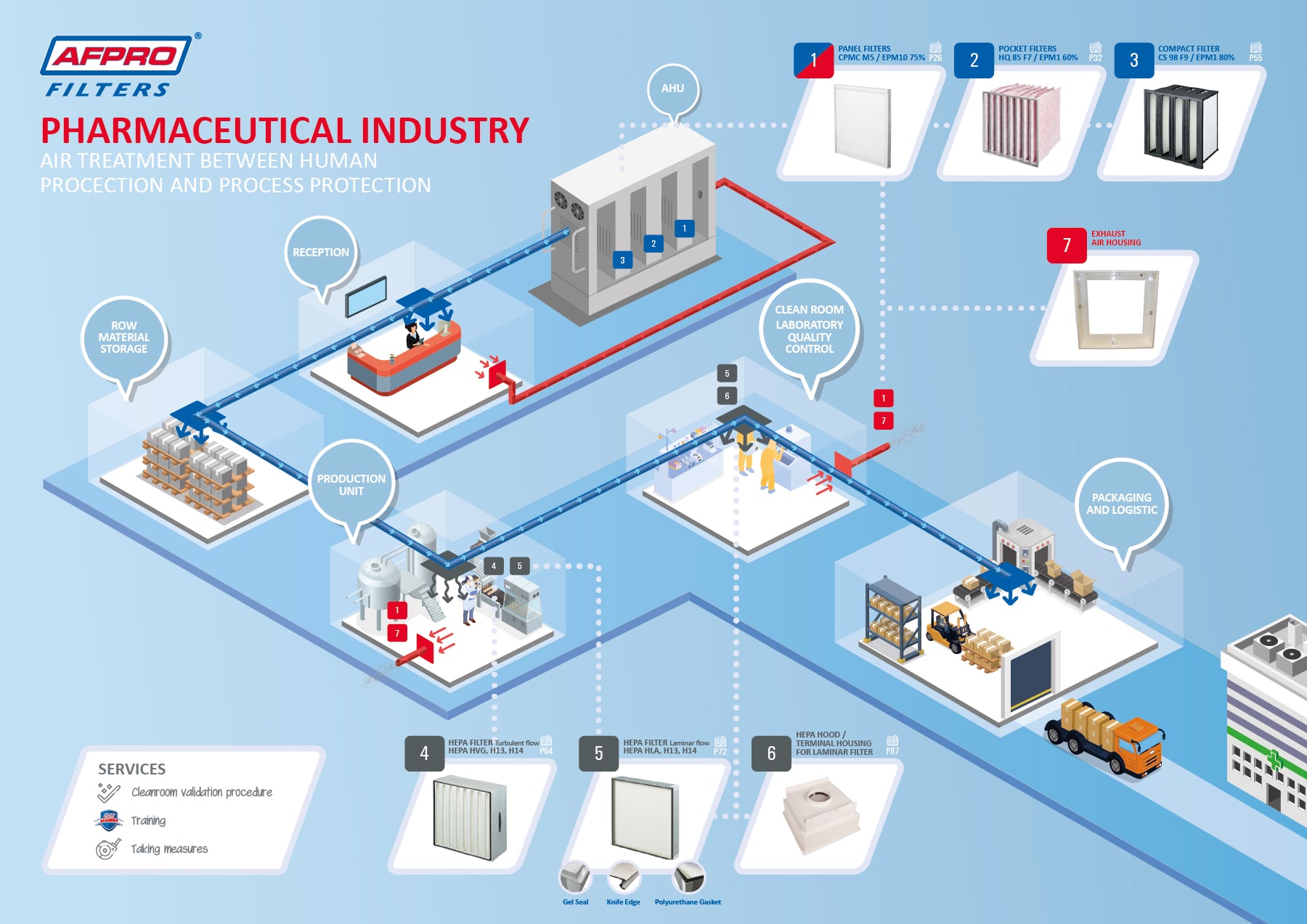
Initial clean room qualification includes in part an assessment of air quality under as built static conditions. Clean room in pharmaceutical manufacturing room is designed and controlled and maintained to attain a highest level of clean environment so as to prevent microbial bacterial and viral and particulate matter contamination. Cleanroom qualifications are a huge investment of a company s money time and resources. Iso 14644 1 2015 cleanrooms and associated controlled environments part 1 2.
Sterile area cleanroom qualification sterile area validation has different tests like air supply air velocity air changes flow pattern filter integrity pressure test particle count temperature recovery test microbial count relative humidity noise level and vibration test. Basic clean room requirements designs for gmp clean rooms what is a clean room. The digital edition is available online.